137
Specific heat
Specific heat of water (4184 J/Kg
0
C) is higher than most of the liquids excepting
ammonia and about 5 times higher than most of common heavy solids like rock,
concrete etc. Higher specific heat mans water heats up slowly and cools over
longer period of time helping moderation of climate near large water bodies.
Thermal fluctuation is also slow, as rapid thermal fluctuation may cause danger
in sustenance of life.
Heat of Vaporisation
Water requires unusually high heat to vaporize (2258 KJ/K
3
). Water vapor this
stores an unusually high energy as heat and transports such high energy from
one place of globe to other areas, keeping our climate suitable for life.
Water as a solvent
Water is a good solvent and dissolves more substances than any other
solvents. Water this transports many nutrients to the soil for organic growth.
Water also eliminates wastes from the earths surface. Water is thus always
contaminated and can also carry hazardous wastes.
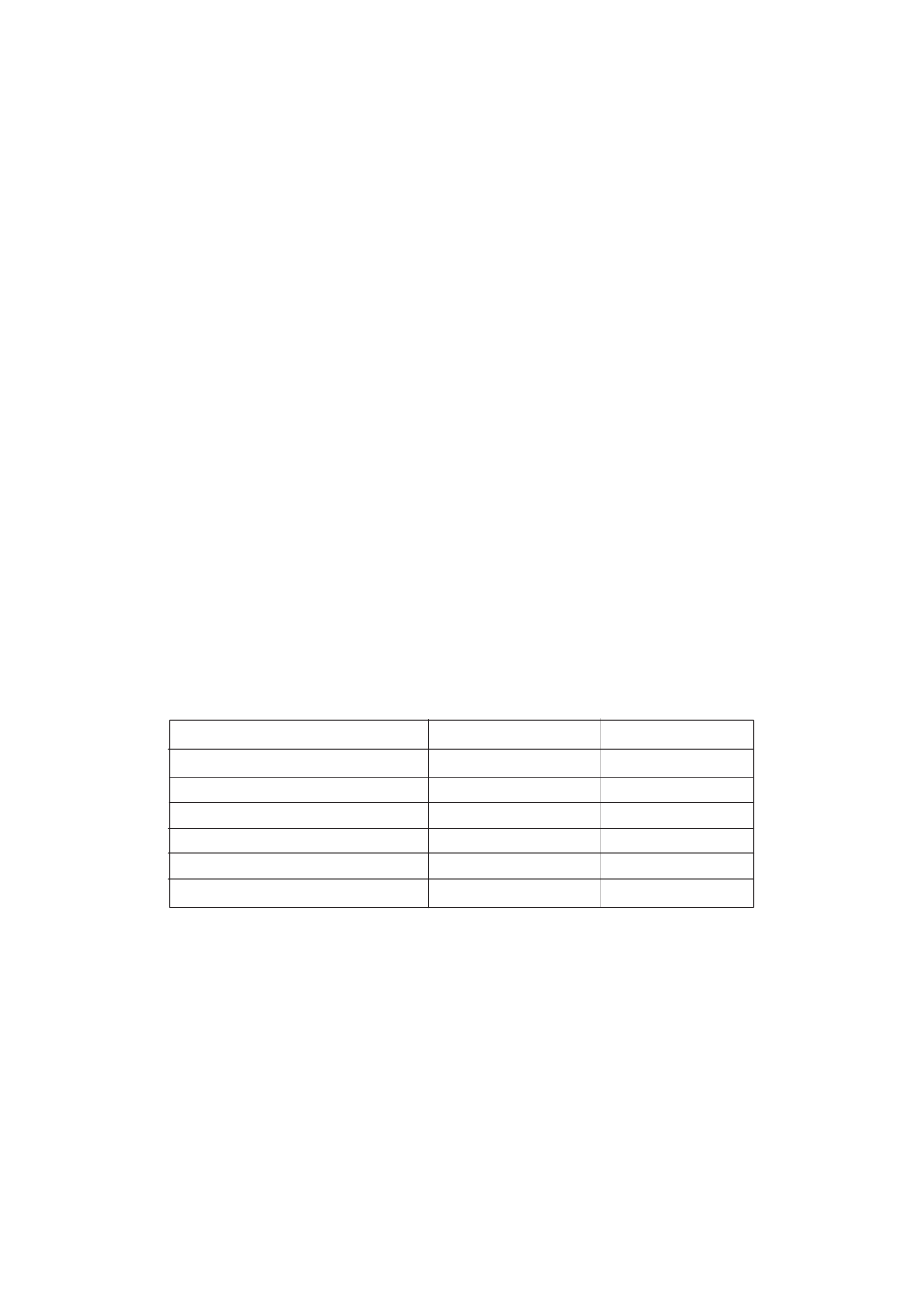
Important Physical Properties of Water
Property
SI Units
U S C S Units
Specific heat (at 17
0
C)
4.184 KJ/Kg
0
C 1.00 Btu/lb
0
F
Latent heat of vaporisation
2258 KJ/Kg
972 Btu/lb
Heat to vaporize 17
0
C water
2459 KJ/Kg
1057 Btu/lb
Latent heat of fusion
333 KJ/Kg
144 Btu/lb
Density at 4
0
C
1000.00 Kg/m3
62.4 lb/ft3
(8 - 34 lb/gal)
Units of Measurement of Water
Water is a liquid and when in static condition is measured in volume. Thus,
when it is stored in a 1 ft x 1 ft x 1 ft pot/cylinder, it is 1 cu.ft. of water in FPS
system or 1 cm x 1 cm x 1 cm pot/cylinder it is 1 C.C. of water in CGS system.


